Colloidal aggregation in drug discovery & drug formulation

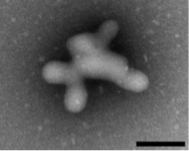
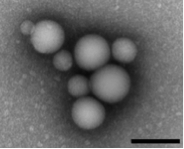
Many organic molecules form colloidal aggregates at micromolar and sub-micromolar concentrations. The colloids are a state intermediate between true solution and precipitate, with properties different from both. They inhibit both soluble and membrane proteins without specificity but with a shared mechanism: direct association and binding followed by partial denaturation. About 2% of compound libraries aggregate at micromolar concentrations, making them the dominant artifact in early drug discovery and chemical biology. Recent work suggests that they are stable in multiple biological media, including cell culture, serum, and simulated intestinal fluid.
In ongoing projects, we are investigating their physical structures and mechanisms of action, and their effects in early discovery, SAR progression, efficacy in cell-culture, and in formulation and delivery. A recent project focuses on opportunities to exploit the unique properties that colloidal aggregates possess, such as the ability to specifically and reversibly sequester folded proteins over DNA and peptides.
Recent papers include:
- D Duan et al. Internal Structure & Preferential Protein Biding of Colloidal Aggregates. ACS Chem. Biol. 12, 282-290 (2017).
- CK McLaughlin et al. Stable Colloidal Drug Aggregates Catch and Release Active Enzymes. ACS Chem. Biol. 11, 992-1000 (2016).
- JJ Irwin et al. An Aggregation Advisor for Ligand Discovery. J Med Chem. 58, 7076-87 (2015).
- SC Owen et al. Colloidal Aggregation Affects the Efficacy of Anticancer Drugs in Cell Culture. ACS Chemical. Biology 7, 1429-35 (2012).
Supported by NIGMS GM71630.